Shaping Immunity in Early-Life with Vaccine Adjuvants
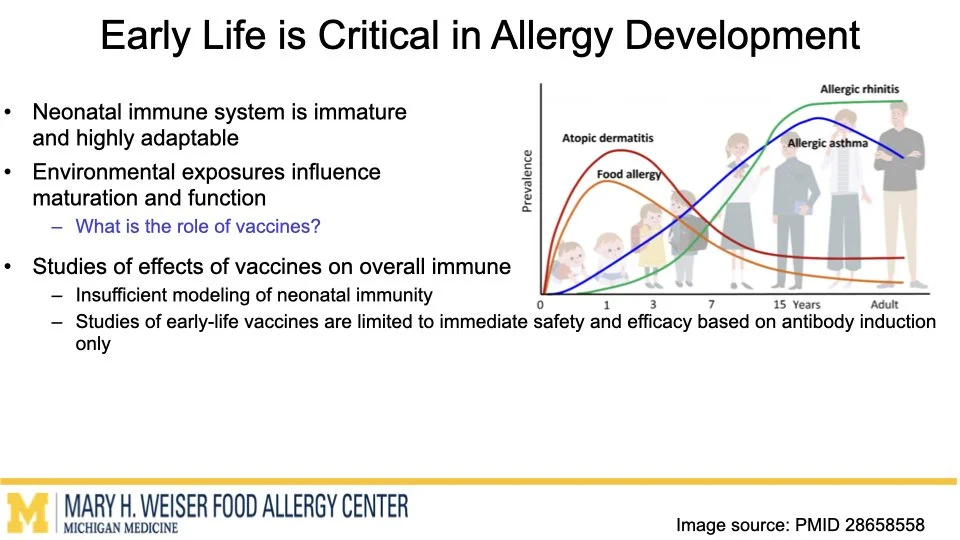
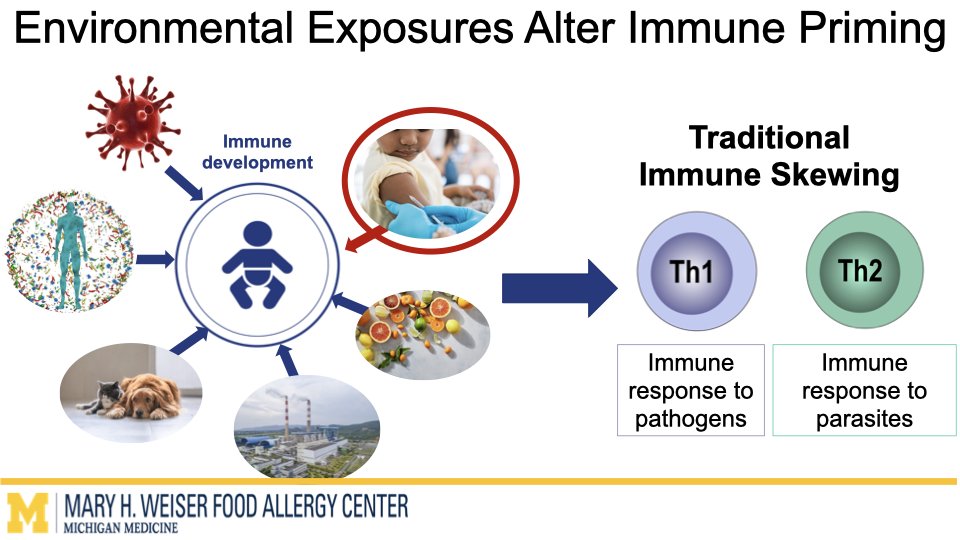
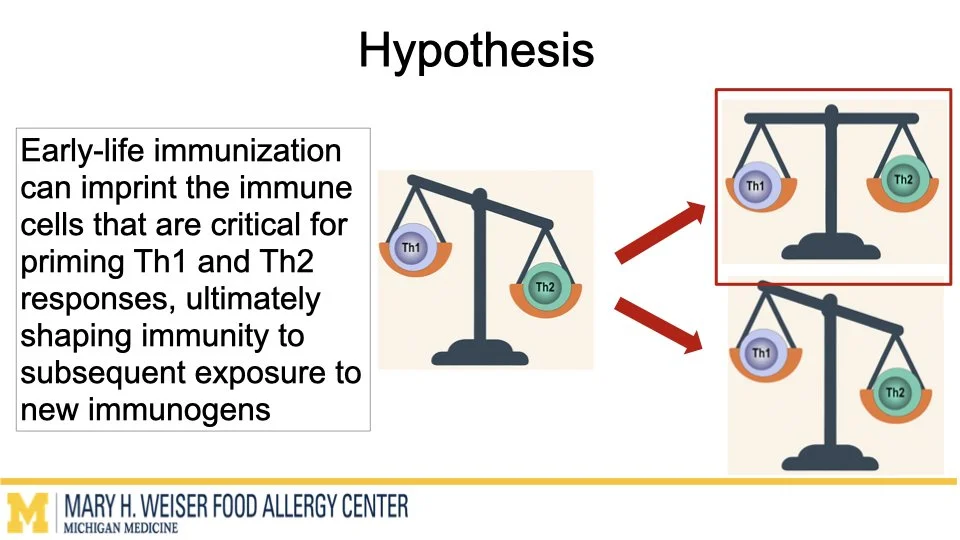
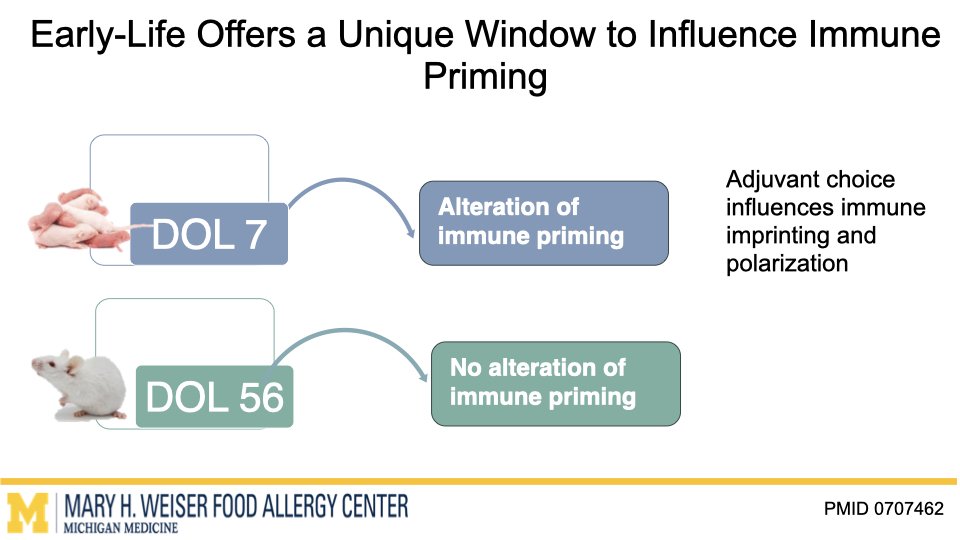
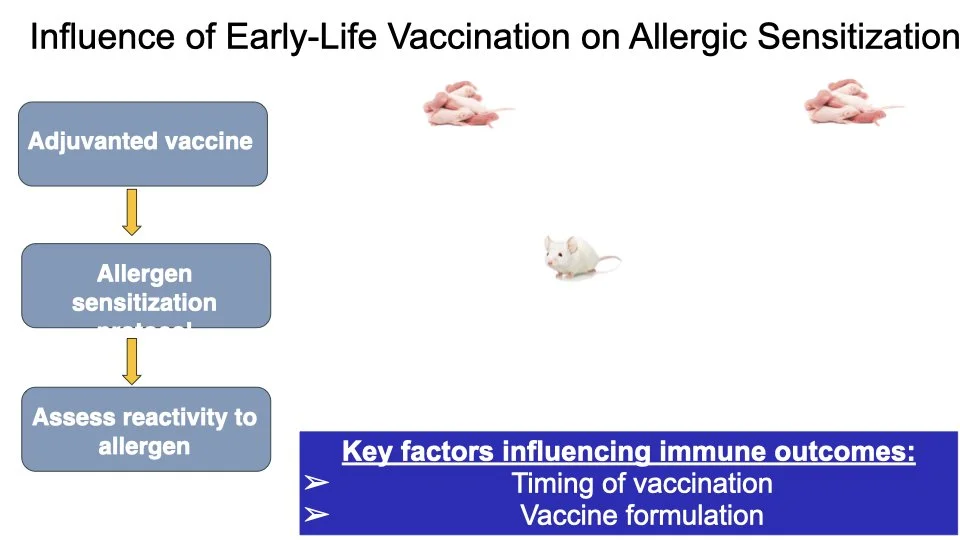
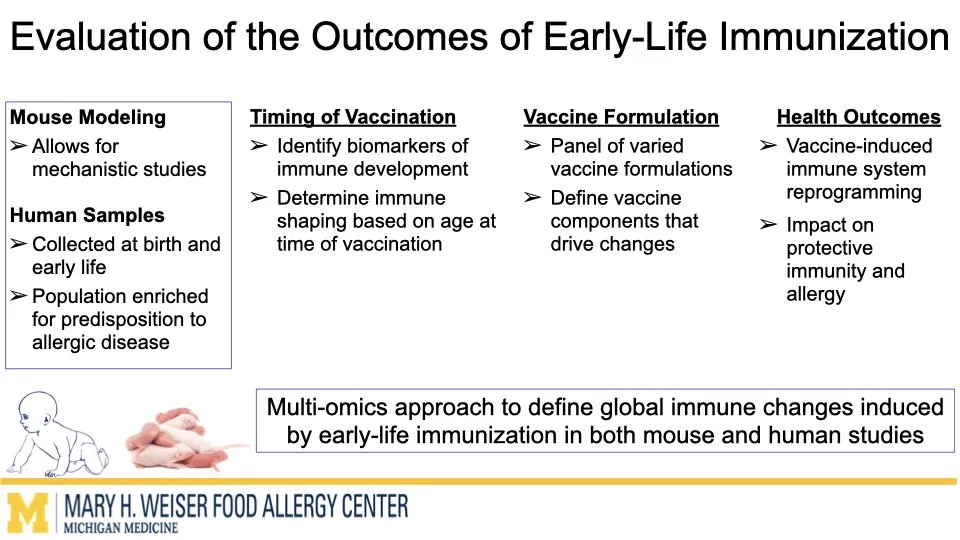
Vaccines are one of the most effective tools to protect human health. Emerging research from our group and others suggests that certain vaccines can do more than help fight infections and may also influence how the immune system responds to other triggers later in life. O’Konek lab is interested in how vaccine components that boost the immune response, commonly called adjuvants, can shape these heterologous effects.
Funded by Food Allergy Fund
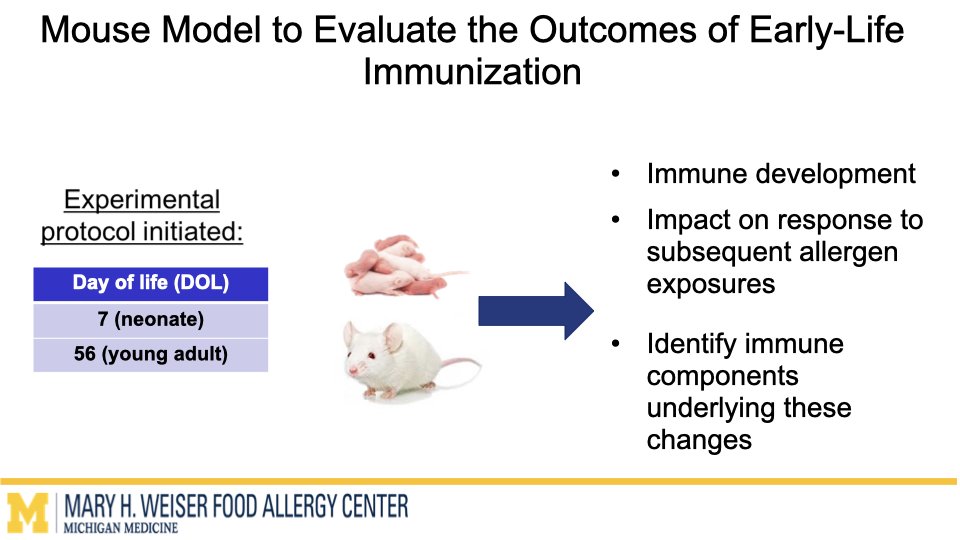
In this project supported by the Food Allergy Fund, we are investigating the differential effects of a panel of diverse vaccine adjuvants across different ages in a mouse model of early-life immunization. By identifying which adjuvants imprint the immune system to alter these broad immune responses, we will select lead candidates for in-depth mechanistic studies, which will utilize a multi-omics approach to examine how and why these changes occur. This stepwise approach will provide vital proof-of-concept data to support future human clinical studies.
This research highlights the importance of understanding vaccines not just as shields against infection but as tools that can help build lifelong health. Ultimately, our work aims to lay the foundation for the design of vaccine strategies that protect from both infectious disease and the growing epidemic of food allergies.
Repurposing metformin to treat food allergy
Anaphylaxis is a rapid, life-threatening allergic reaction that can be triggered during oral food challenges (OFCs)—the gold standard for diagnosing food allergies. However, current diagnostic methods rely heavily on subjective observation and patient reporting, which are particularly limited in infants, toddlers, and anxious children.
Funded by Food Allergy Fund
These goals led us to consider the use of established drugs that are safe, inexpensive, and potentially able to ameliorate allergy to multiple foods. Metformin use is associated with decreased asthma prevalence and exacerbation. In addition, Dr. Fred Finkelman reported that metformin prevented food allergy development and suppressed established food allergy to cow’s milk, egg and peanut in mouse models. We propose that metformin, likely acting through the unfolded protein response, has the potential to treat established food allergy. Metformin is FDA-approved and has significant safety data in children; however, it has not been studied in patients with food allergy. The Food Allergy Fund will support a pilot study using Metformin to treat food allergies to determine safety and efficacy for future scaled clinical trials.
Dietary Interventions to Mitigate Microbiome-mediated Food Allergic Responses
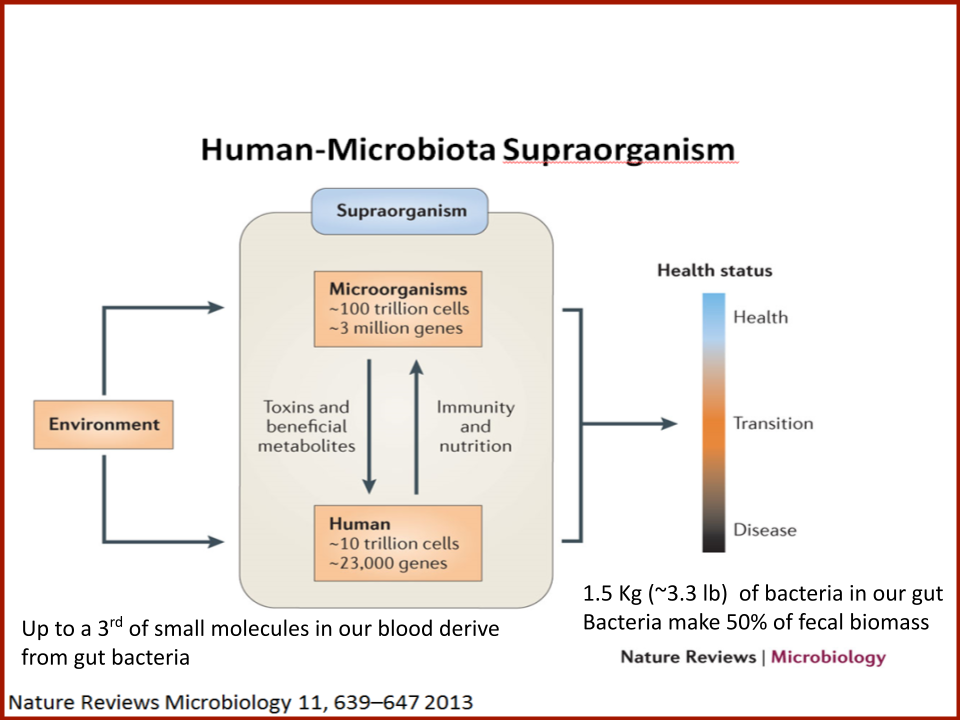
Thirty-three million Americans, or one-in-ten adults and one-in-thirteen children, have IgE-mediated food allergies. Studies from our group and others have linked gut microbiome composition and, more specifically, microbial metabolic dysfunction with food allergy.
Diet profoundly impacts gut microbiomes and their metabolic productivity. However, consumption of identical meals or even specific dietary substrates leads to distinct metabolic products, the variance of which relates to fecal microbiome function. Our previous studies demonstrated that specific microbial-derived metabolites influence allergic inflammation. Yet, how food allergic gut microbiomes process dietary substrates to produce metabolites that shape immune function and hyper-responsiveness to food allergens is unknown, and whether dietary substrates can be used to mitigate food allergic immune responses remains entirely unexplored. We hypothesize that microbial metabolism of specific dietary substrates promotes hyper-responsive immunity to food allergens, while others mitigate allergic response and aim to identify those that reproducibly suppress allergic response. Thus, we propose to leverage a large (n=127) collection of banked fecal samples from peanut allergic patients to model diet by gut microbiome interactions in vitro to identify dietary substrates that reduce immune responsiveness to peanut allergen. If successful, this approach will be expanded to include patients of varying ages with additional food allergies e.g. milk, egg or tree nut, data that will be fed into our model. Addition of such data would improve prediction accuracy and facilitate our ultimate aim of precision nutritional interventions to manipulate the gut microbiome and mitigate food allergic responses, particularly in those with severe disease.
Develop an AI Food Allergy Diagnostics Platform for Patient Care and Treatments
We aim to develop a multimodal AI platform for food allergy diagnostics that integrates diverse data sources, including skin tests, blood tests, molecular data, histological data, and medical history.
Funded by Food Allergy Fund
We aim to develop a multimodal AI platform for food allergy diagnostics that integrates diverse data sources, including skin tests, blood tests, molecular data, histological data, and medical history. This platform will leverage advanced machine learning algorithms to analyse and correlate these varied datasets, providing a comprehensive and nuanced understanding of food allergies. Initial findings suggest that our pipeline, which integrates physiological markers across multiple data sources, can predict oral food challenge outcomes months in advance of the challenge. In addition, our causal learning model improves decision-making by informing recommendations on whether to continue or discontinue oral immunotherapy. By incorporating the different data modalities, the AI system will identify complex patterns and interactions that single-modal approaches might miss. This integration will enable more accurate and personalized diagnostics and allow for earlier and more precise identification of food allergies.
Discovering New Immune Pathways
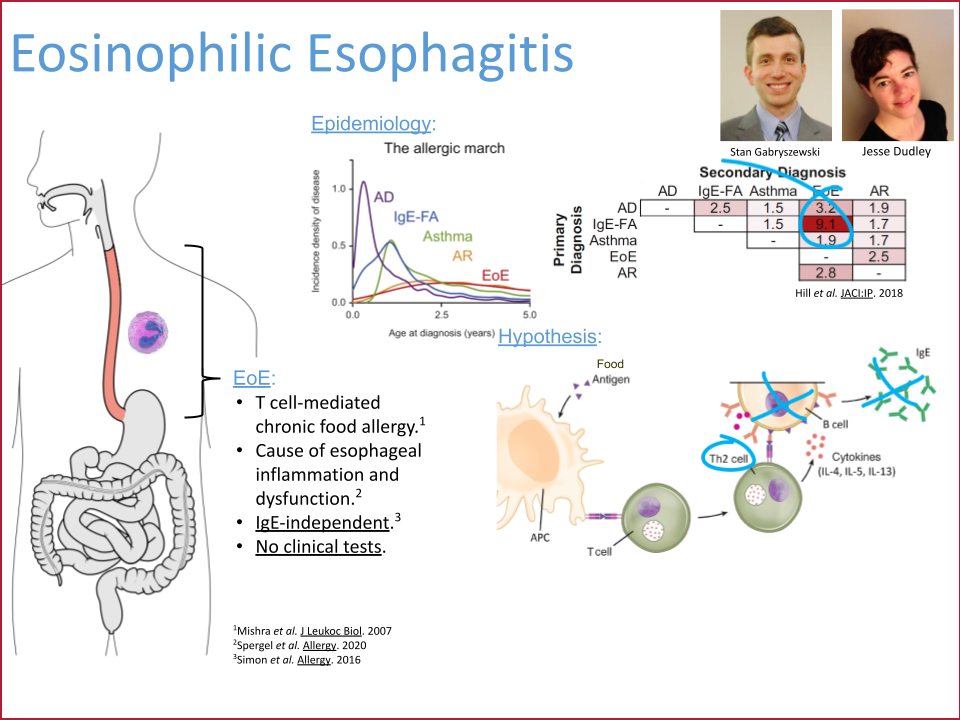
Dr. David A. Hill (Children’s Hospital of Philadelphia) and his team have uncovered a surprising immune pathway in EoE: a typically non-allergic cytokine, Interferon gamma, disrupts the function of protective regulatory B cells. This breakthrough challenges conventional thinking and could pave the way for entirely new treatment strategies—translating cutting-edge science into real-world care.
Funded by Food Allergy Fund
The study's findings offer a new perspective on the immunological underpinnings of EoE and potential therapeutic strategies. By identifying IFNγ as a key driver of Breg dysfunction, the work suggests that targeting this cytokine could restore Breg-mediated regulation and mitigate inflammation. These insights not only deepen our understanding of EoE's pathogenesis but also open the door to innovative treatments that harness immune regulatory mechanisms to manage the disease more effectively.
Wearable Anaphylaxis Detection for Improved Safety and Phenotyping
Anaphylaxis is a rapid, life-threatening allergic reaction that can be triggered during oral food challenges (OFCs)—the gold standard for diagnosing food allergies. However, current diagnostic methods rely heavily on subjective observation and patient reporting, which are particularly limited in infants, toddlers, and anxious children.
Funded by Food Allergy Fund
Understanding the Burden of Multi-Food Allergy and Allergic Comorbidities in U.S. Children
We found that FMT increases the threshold dose at which a subset of patients reacts to peanut. These patients were initially reacting to traces of peanut and their threshold dose when up to 2.5- 3 peanuts, after one FMT only. This improvement lasted at least 4 months.
Funded by Food Allergy Fund
Our team at Northwestern University and Lurie Children’s Hospital of Chicago has led a multi-site, collaborative effort to recruit and follow a large cohort of English- and Spanish-speaking children with food allergy for the past 7 years—carefully assessing their food allergy status via clinical visits, quarterly surveys, blood, skin and stool microbiome collection, as well as regular extraction of electronic health record data. Together these data allow us to paint a much more complete picture of the allergic disease burden experienced by pediatric food allergy patients in the US, who appear to be at elevated risk of many related conditions, including (but not limited) to atopic dermatitis, asthma, environmental allergies, eosinophilic esophagitis, oral allergy syndrome, and chronic urticaria.
The Food Allergy Fund is supporting systematic efforts to better characterize the degree to which these chronic allergic conditions co-occur, as well as understand modifiable determinants of allergic disease burden that can be targeted by scalable interventions. One advantage of the assembled NIAID-supported longitudinal cohort is its very large (N>1500) sample of parents and children who span the entire pediatric age spectrum, from infancy to young adulthood. This allows examination of not only the early life factors that are increasingly acknowledged to play key roles in the development of multiple allergic conditions but also factors that are present throughout childhood that may also be disease modifying. Preliminary findings from these FAF-supported analyses presented at the 2025 Food Allergy Fund Summit identify remarkably high rates of multi-food allergy and allergic comorbidities in this large, geographically, racially/ethnically and socioeconomically diverse cohort of children with allergist-confirmed FA. Furthermore, these analyses highlight a potentially causal role of greater eczema severity and duration, not only in infancy, but into mid- and later childhood. Specifically, children with more severe and persistent eczema appear to be at substantially greater risk of multi-food allergy, as well as development of additional allergic diseases as they age. Ongoing work seeks to further clarify these associations, as well as further understand the growing burden and etiology of multi-food allergy and related chronic conditions in the US population.
Metabolomic and Metagenomic Insights into FMT Response in Food Allergy
Dr. Rima Rachid and Dr. Talal Chatila of Boston Children’s Hospital as Principal Investigators
Funded by the Food Allergy Fund
We then performed a fecal transplant in mice that are highly prone food anaphylaxis. In the mice that received the human stools from responders’ patients prior to FMT, there was no protection from anaphylaxis. In the mice that received the human stools post FMT, there was strong protection from anaphylaxis. This was not observed when we used the stools of non responders’s patients.
The protection in the mice was accompanied by a very strong increase (1000 fold) in the abundance of Bacteroidales species in the stools. We have found before (Nature Medicine 2019) that Bacteroidales are protective against food allergy. We also found that there is a metabolomic signal in the human patients and the mice that responded, suggesting that the bile acid metabolites are critical for the protective response. When using a Bacteroidales strain that does not metabolize bile acid, we did not find any protection from anaphylaxis in mice.







Food Allergy Fund Launches Exclusive Research Roadmap to Accelerate Food Allergy Prevention and a Cure
The Food Allergy Fund (FAF), the leading nonprofit that funds food allergy research, has marked a significant milestone in the field: A first-ever Food Allergy Research Roadmap, a comprehensive guide designed to accelerate medical discovery and development progress within the next five years.













New York, NY (May 31, 2024) – The Food Allergy Fund (FAF), the leading nonprofit that funds food allergy research, has marked a significant milestone in the field: A first-ever Food Allergy Research Roadmap, a comprehensive guide designed to accelerate medical discovery and development progress within the next five years. FAF has brought together an exclusive working group of renowned scientists and allergists to identify the root causes of food allergies and focus on cutting-edge approaches to prevent, treat, and cure the disease. In addition, FAF’s Scientific Advisory Board is an unparalleled group of doctors and scientists from leading research institutions across the country.
While current food allergy research primarily targets disease symptoms and single-food immunotherapy treatments, FAF's Research Roadmap adopts a transformative approach. It prioritizes prevention and cures for multiple food allergies and allergic diseases, developing disease-modifying therapeutics, and catalyzing multiple proof-of-concept clinical trials. The FAF Research Roadmap will serve as a scientific business plan for scientists and funders.
Over the next five years, FAF will focus on eliminating anaphylactic reactions, inducing and maintaining immune tolerance to foods, and preventing food allergy in the next child or adult.
FAF's vision includes:
Forging partnerships between academia and industry
Utilizing AI-driven platforms
Recruiting new talent and medical disciplines
Hosting curated research retreats
Repurposing therapeutics
Creating a venture philanthropy platform to address the funding gap
Today, food allergies are a growing concern and a pressing public health issue. The incidence and severity are rising, with 1 in 10 adults and 1 in 13 children affected. More than forty percent of people with food allergies are allergic to multiple foods, and every three minutes, a person visits the emergency room due to an allergic reaction.
"The food allergy community has never had a real-time tool like this roadmap, and it is an essential next step in hastening our progress to find a cure for the disease," said Ilana Golant, founder and CEO of the Food Allergy Fund. "To end food allergies, we have assembled the brightest minds in America to help us map our goals, pool resources, and collaborate across disciplines, proactively changing the research culture."
The ground-breaking Research Roadmap was unveiled at the 9th Food Allergy Fund Summit, which brings together leading experts and advocates. The marquee summit, held at the Paley Center for Media in New York during Food Allergy Awareness Month in May, serves as a platform for sharing knowledge, fostering collaboration, and driving innovation in food allergy research.
Research Roadmap Working Group
Dr. Richard Insel, Research Professor, Department of Pediatrics, University of Rochester School of Medicine & Dentistry
Stephanie Eisenbarth, MD, PhD, Director, Center for Human Immunobiology; Chief of Allergy and Immunology in the Department of Medicine; Roy and Elaine Patterson Professor of Medicine; Professor of Medicine (Allergy and Immunology) and Pathology, Northwestern University
Dr. Rima Rachid, Director of Allergen Immunotherapy, Allergy and Asthma Program; Co-Director, Food Allergy Program, Boston Children’s Hospital; Attending Physician, Division of Immunology; Associate Professor of Pediatrics, Harvard Medical School
Dr. Marc Rothenberg, MD, PhD, Director, Division of Allergy and Immunology; Director, Cincinnati Center for Eosinophilic Disorders, Cincinnati Children’s Hospital
Scientific Advisory Board
Martin J. Blaser, M.D., Henry Rutgers Chair of the Human Microbiome; Professor, Department of Medicine and Microbiology; Director, Center for Advanced Biotechnology and Medicine RBHS, Robert Wood Johnson Medical School
Scott Boyd, M.D., Ph.D., Director, Boyd Lab for Human Immunology Member; Child Health Research Institute Member; Sean N. Parker Center for Allergy & Asthma Research; Associate Professor of Pathology Stanford University School of Medicine
Linda Herbert, Ph.D., Director, Psychosocial Clinical Program, Division of Allergy & Immunology; Assistant Professor, Division of Psychology & Behavioral Health Children's National Health System
Wayne I. Lencer, M.D., Chief, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital; Director, Harvard Digestive Diseases Center; Longwood Professor of Pediatrics, Harvard Medical School
J. Christopher Love, Ph.D., Professor of Chemical Engineering, Massachusetts Institute of Technology (MIT); Associate Member, Broad Institute; Associate Member, Ragon Institute of MGH, MIT and Harvard
Rachel L. Miller, M.D., Merksamer Professor in Immunology; Chief, Division of Clinical Immunology, Icahn School of Medicine at Mount Sinai
Kari Nadeau, M.D., Ph.D., Chair, Department of Environmental Health, Harvard T.H. Chan School of Public Health
Noah Wolcott Palm, Ph.D., Assistant Professor of Immunobiology, Yale University School of Medicine
Hugh A. Sampson, M.D., Kurt Hirschhorn Professor of Pediatrics; Director Emeritus, Jaffe Food Allergy Institute; Past President, American Academy of Allergy, Asthma & Immunology, Icahn School of Medicine at Mount Sinai
Wayne G. Shreffler, M.D., Ph.D., Chief, Pediatric Allergy & Immunology; Director, Food Allergy Center, Massachusetts General Hospital; Associate Professor of Pediatrics, Harvard Medical School
Scott H. Sicherer, M.D., Director, Elliot and Roslyn Jaffe Food Allergy Institute; Chief, Division of Allergy and Immunology, Department of Pediatrics, Icahn School of Medicine at Mount Sinai
Marsha Wills-Karp, Ph.D., Co-Chair, Consortium of Food Allergy Research (CoFAR) National Institutes of Health (NIH); Chair, Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health
Gary D. Wu, M.D., Director, Molecular Biology Core; Associate Chief for Research, Division of Gastroenterology; Associate Director, Center for Molecular Studies in Digestive and Liver Disease; Ferdinand G. Weisbrod Professor in Gastroenterology, Hospital of the University of Pennsylvania
###
Media Contact: Amy DiElsi, adielsi02@gmail.com, 215-990-3006
The Food Allergy Fund is the leading nonprofit dedicated to funding food allergy research. FAF's grants support the creation of new treatments that will address the root causes of food allergies. Through our groundbreaking research and unique thought leadership summits, we accelerate innovation to find solutions. To learn more, visit www.foodallergyfund.org.
Repurposing Zileuton to prevent anaphylaxis to food allergens by reducing allergen absorption
To avoid potentially life-threatening anaphylaxis, individuals with food allergy must avoid the allergen(s) they are sensitized to. Treatment options to prevent anaphylaxis from accidental or therapeutic allergen ingestion remain limited. The goal of our lab is to identify new therapeutic interventions to prevent allergic reactions to food based on fundamental mechanistic studies.
Funded by Food Allergy Fund
While food-specific IgE is necessary for anaphylaxis, it is not sufficient; some individuals with food-specific IgE do not experience allergic symptoms upon ingesting the allergen(s) they are sensitized to. This unresponsive state has been termed “sensitized tolerant” and suggests that other factors, such as intestinal absorption of allergens, could play a role in symptomatic food allergy. It is important that allergens are absorbed in an intact form in order to be recognized by the immune system and trigger anaphylaxis. Our work and previous work in the field has shown that most people absorb detectable levels of intact food allergens after ingestion, but the amount varies person to person. We discovered that the degree of food allergen absorption correlates with anaphylaxis in a murine food allergy model and that this absorption could be reduced acutely with the FDA-approved drug, Zileuton. We propose that such drugs have the potential to prevent anaphylaxis in patients with food allergy. Although some of these agents are FDA-approved, they have not been used in patients with food allergy.
This Food Allergy Fund award will support a proof-of-concept study to determine whether intact food allergen absorption can be acutely reduced in adults with food allergy. Our study will enroll 21 adults and compare each participant’s absorption of an ingested protein allergen that they are not sensitized to with and without Zileuton pre-treatment. This will enable us to determine whether we can block uptake of intact ingested proteins in humans. The outcome of this study will establish the rationale for a future intervention trial to determine whether we can prevent anaphylaxis in individuals with food allergy.
Repurposing Abrocitinib for Food Allergy
Identification of an allergen agnostic therapeutic that could reduce reaction risk by improving allergen threshold, reducing reaction severity, or providing an adjuvant effect to enhance the safety and efficacy of oral immunotherapy, would be a major advance for food allergy treatment. We propose that repurposing a JAK inhibitor, specifically Abrocitinib, for food allergy could meet this goal.Janus kinase (JAK) inhibitors are a type of medication that treat diseases by blocking JAK enzymes that send signals that start inflammatory immune processes.
Funded by Food Allergy Fund
Abrocitinib is a specific JAK1 inhibitor that is already approved for adults and adolescents with atopic dermatitis and well-tolerated. It is administered orally, does not cause immunogenicity, and is not allergen-specific, offering the possibility of using one medication to treat multiple allergies
We are currently conducting a mechanistic pilot study of Abrocitinib in food allergy (NCT05069831). This study is enrolling up to 40 adults to complete 4 months of treatment with Abrocitinib to examine the effect of Abrocitinib on basophil activation tests, allergen-specific IgE, and skin prick test wheal size.
This award from the Food Allergy Fund will support extended mechanistic studies on samples collected in our pilot study to further understand the effect of JAK1 inhibition on food allergy. We will use these samples to sensitize basophils and mast cells in vitro and explore how Abrocitinib impacts the milieu of antibodies, cytokines, chemokines, and other activating factors that may inhibit basophil and mast cell activation. We will also examine how Abrocitinib affects allergen-specific IgE and IgG4 antibodies and pro-allergic B cell phenotype.
mRNA Treatment for Allergic Response
Marc E. Rothenberg MD, PhD, Professor of Pediatrics, Bunning Chair of Allergy and Immunology, Director, Division of Allergy and Immunology, Director, Cincinnati Center for Eosinophilic Disorders Director, Cincinnati Children’s Hospital Medical Center as Principal Investigator
Exclusively Funded by the Food Allergy Fund
In our initial studies, model allergens have been incorporated into mRNA vaccines, starting with the egg protein ovomucoid (OVA) and a specific immunogen from peanuts (Arah2). These vaccines, referred to as allergen-mRNA vaccines, are being examined in pre-clinical models of allergic diseases, including asthma, food allergy, and anaphylaxis. Early studies have demonstrated the powerful ability of allergen-mRNA vaccination to change allergic responses, including lowering allergy-driving antibodies (IgE), shifting allergic adaptive immunity away from a harmful response (e.g., shifting type 2 to type 1 responses), and lowering allergic inflammatory cells, such as eosinophils. At this stage, the experiments are focused on proof-of-concept pre-clinical studies, including experiments designed to deeply investigate how these mechanisms work. Collectively, mRNA vaccines hold great promise for the treatment of allergic diseases, and experiments are underway to make this promise a reality as quickly as possible.
A Phase II Trial Evaluating the Safety and Efficacy of Microbiota Transplantation Therapy (MTT) in Children with Food Allergy
Dr. Rima Rachid and Dr. Talal Chatila of Boston Children’s Hospital as Principal Investigators
Funded by the Food Allergy Fund
With the promising results of Phase One, the Food Allergy Fund is now supporting the Phase II trial -- launched in June 2023. This cutting-edge study, led by Dr. Rachid, evaluates the safety and efficacy of oral encapsulated microbial transplantation therapy (MTT) in teens (age 12-17 years) with peanut allergy, in collaboration with Dr. Alexander Khoruts from the University of Minnesota. MTT is a purified and concentrated form of FMT, containing less than 1% fecal material in the capsules. MTT can be kept in a regular home refrigerator, which offers a “realistic” approach for the future use of FMT. Patients will receive MTT or placebo for one month and will return to have their peanut allergy evaluated to see if their threshold of reactivity has improved.
A “Synbiotic” for the Prevention of Food Allergy
Cathryn R. Nagler, Ph.D.,Pritzker School of Molecular Engineering and Departments of Pathology, Pediatrics and Medicine Biological Sciences Division, University of Chicago as Principal Investigator
Funded by the Food Allergy Fund
There is significant clinical interest in developing live biotherapeutic products (LBPs), from specific commensal species to prevent or treat various disease indications. LBPs are becoming increasingly common; however, they often exhibit limited engraftment. To overcome this limitation, many clinical (and pre-clinical) studies require a pre-treatment course of antibiotics, further depleting the resident microbiota in potentially adverse ways. We propose an alternative strategy: co-delivery of the LBP with prebiotics, i.e., as a synbiotic, to create an ecological niche in the gut to maximize engraftment and butyrate production. We have isolated a novel strain of A. caccae, A. caccae_lahuc, from the feces of a healthy infant and demonstrated that co-delivery of this isolate with a prebiotic (i.e., as a synbiotic) prevents food allergy in mice with a dysbiotic human microbiota. In this proposal we will screen a variety of clinically relevant prebiotics in vitro and in vivo to optimize our synbiotic formulation for maximal efficacy and translatability. We will also examine the mechanisms by which our synbiotic(s) prevent or treat food allergy.
Synbiotic Therapy modifies the fecal microbiome and regulates host immunity to prevent and treat food allergy
Functional T Cell Assays for Food Allergy
Dr. David Hill, Perelman School of Medicine at the University of Pennsylvania, as Principal Investigator
Funded by the Food Allergy Fund
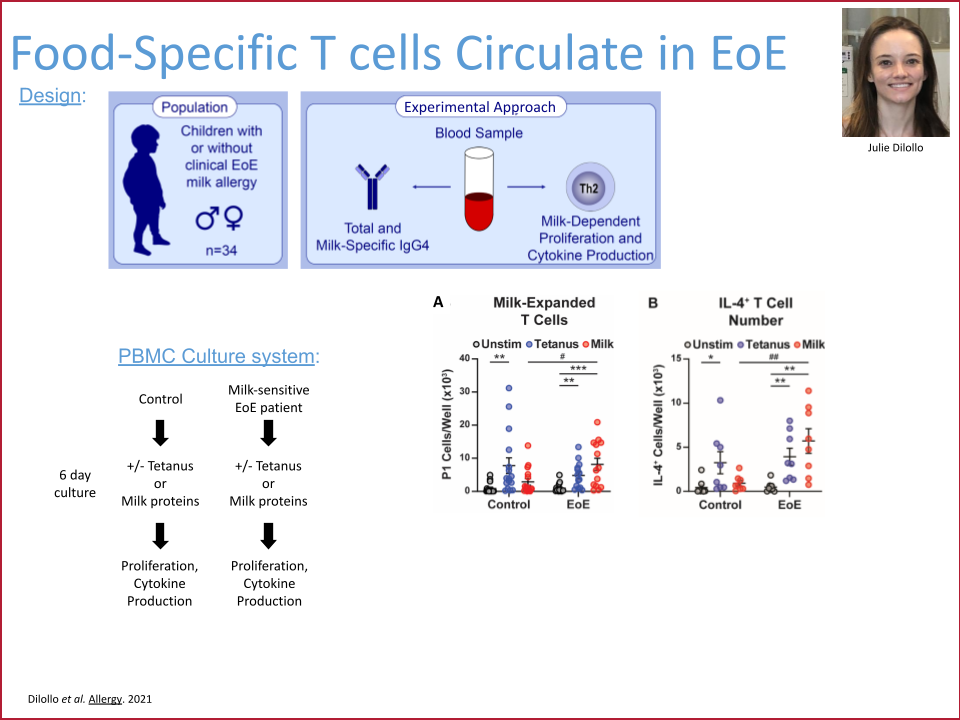
This project aimed to develop functional T cell blood assays for use in the diagnosis, management, and monitoring of food allergy. The promise of such clinical tests was to significantly reduce the time to identify and to determine dietary removal of allergenic foods for non-IgE-mediated food allergies such as eosinophilic esophagitis (EoE) and food protein-induced enterocolitis (FPIES). These tests would also increase the ability to monitor pathogenic and regulatory T cell responses during oral immunotherapy.
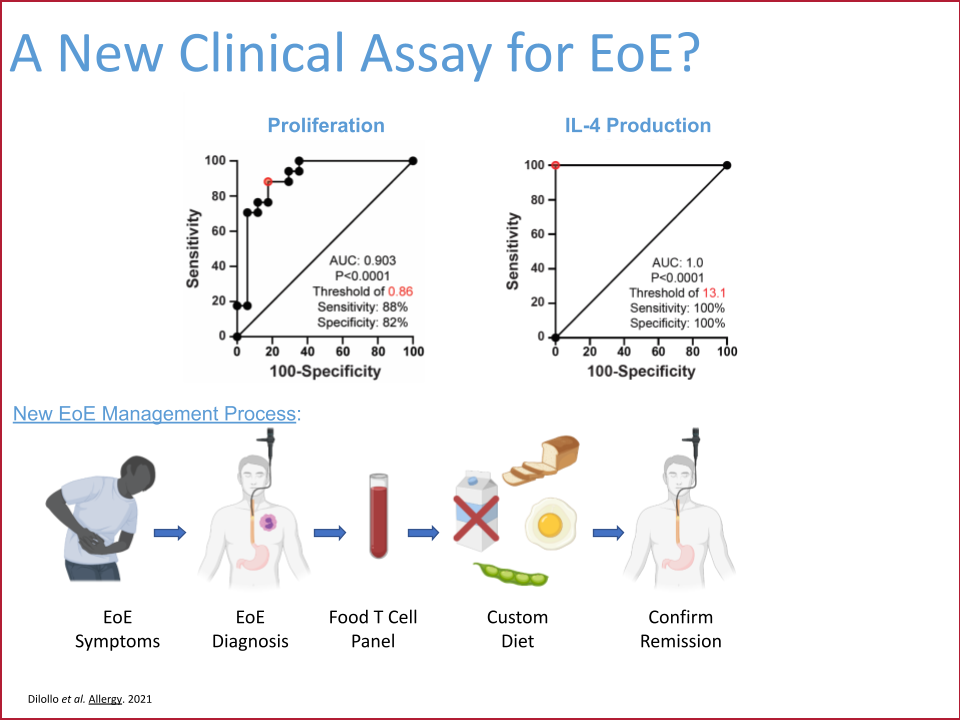
This project completed in December 2024 and successfully developed a minimally invasive blood test that accurately determines the contribution of milk to a patient’s EoE food allergy. This test can be performed on a single tube of peripheral blood, results in six days, and has 100% sensitivity and 80% specificity for presence or absence of EoE milk allergy. The EoE milk allergy blood test has completed development in the Hill Lab and has been transitioned to the CHOP Clinical Immunology Lab. The plan is for it to be offered to CHOP patients in late 2025.
Next steps: EoE soy, wheat, and egg allergy blood tests
Based on the successful approach used to develop the EoE milk allergy test, the Hill Lab has developed similar blood tests for soy, wheat, and egg. Effectiveness of these new tests in patients with confirmed EoE food allergy needs to be tested. A prospective interventional trial is planned which will enroll CHOP EoE patients, perform the soy, wheat, and egg tests (along with the milk test), and provide the results of this testing to the managing CHOP physician. The physician will then integrate the results of the blood testing with other clinical information and work with the patient to develop a personalized elimination diet. After a period of food avoidance, success of this elimination diet will be evaluated by clinical endoscopy. Ultimately, this trial will provide critical pre-clinical data to allow for transition of the soy, wheat, and egg EoE blood tests to clinical use and hopefully ultimately replace the need for endoscopy for diagnostic purposes.


Novel Mechanisms of Blocking Anaphylaxis to Food Allergens
Dr. Stephanie Eisenbarth, Yale School of Medicine and Dr. Adam Williams, The Jackson Laboratory, as Co-Principal Investigators
Funded by the Food Allergy Fund
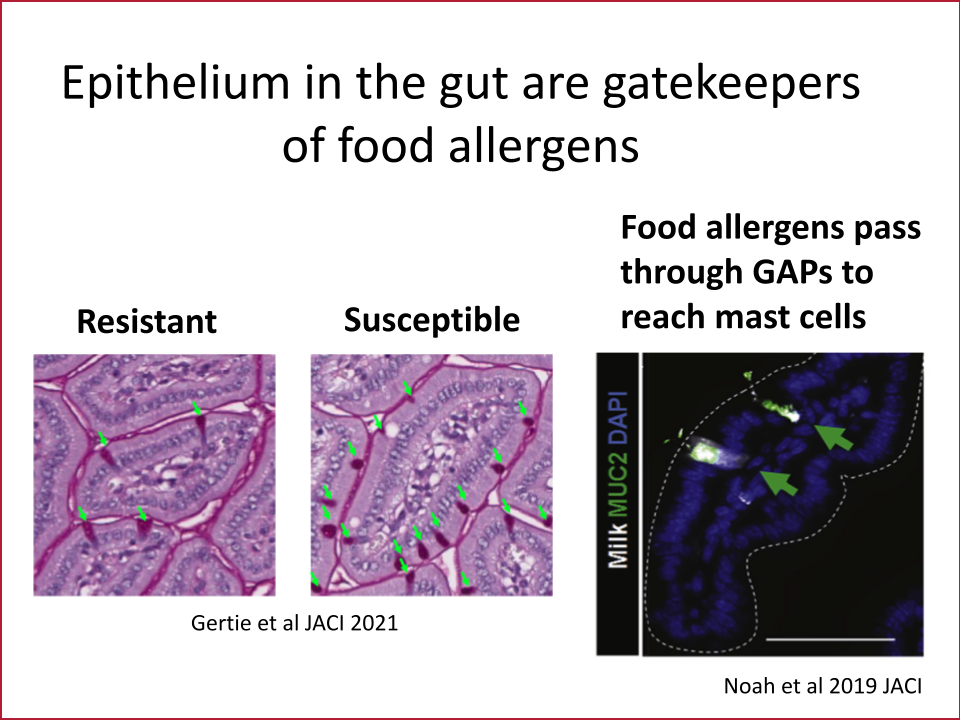
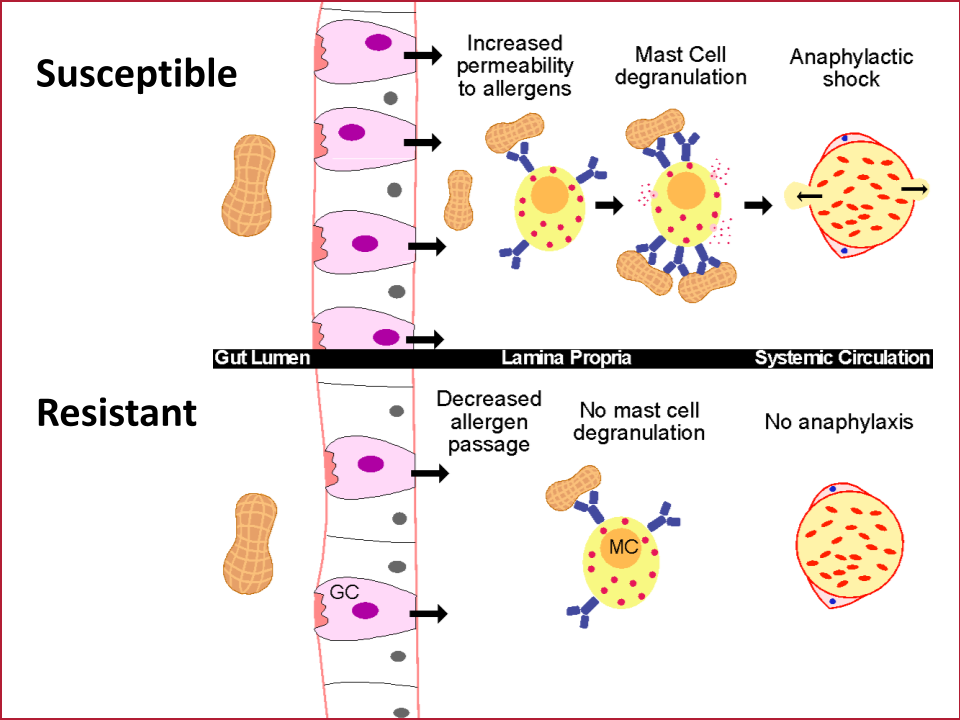
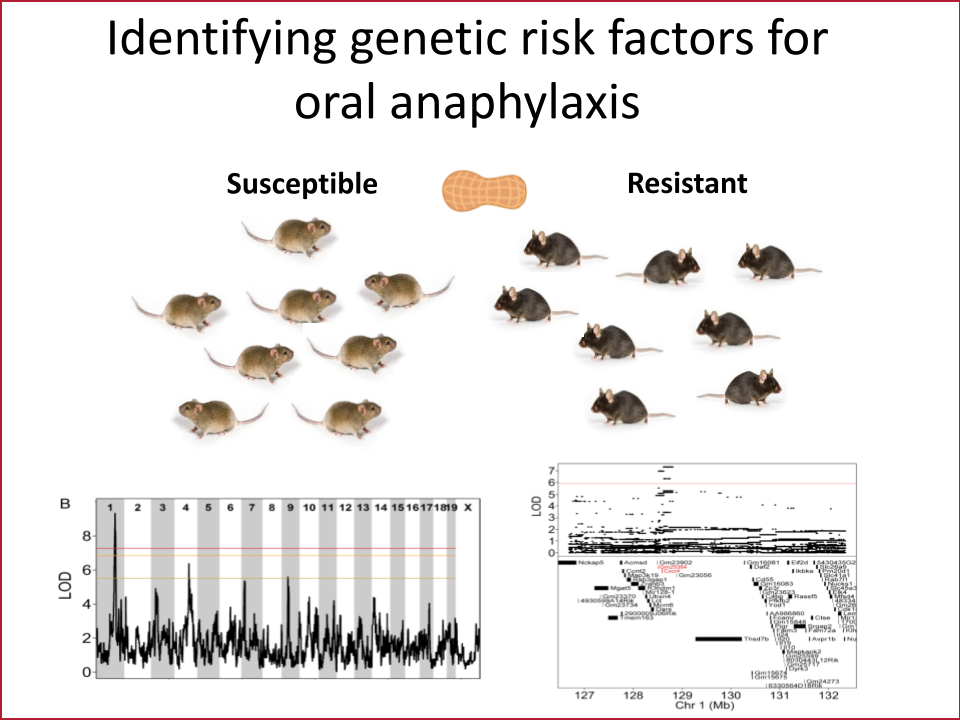
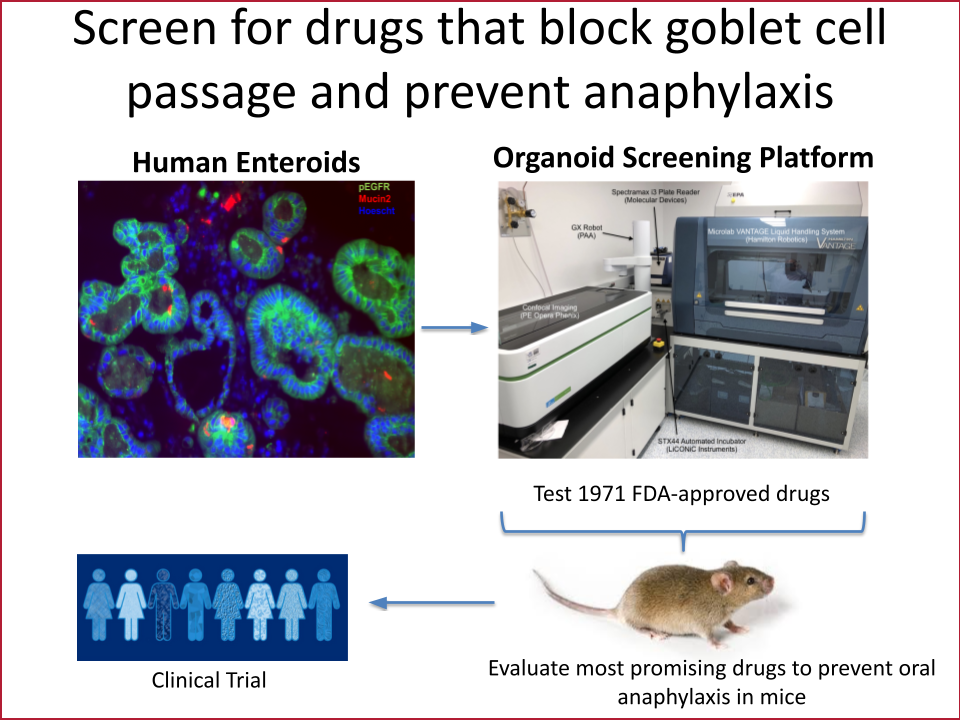
The gut epithelium is a single layer of cells that has the daunting task of absorbing nutrients while keeping pathogens and other potentially harmful things that we have eaten out of our bodies. This cell layer in the intestine is therefore one of the primary barriers keeping intact food allergens from triggering the immune cells that cause anaphylaxis. Our lab’s overarching goal is to discover pathways that are dysregulated in the intestinal barrier in those with anaphylaxis to food allergens. Using biopsies from patients, a unique system to grow gut epithelium in a dish called enteroids and mouse models of anaphylaxis to food, we have discovered new molecules and genes that regulate allergen transport in the gut. We aim to use this knowledge to identify new drug candidates that block allergen transport and therefore anaphylaxis.


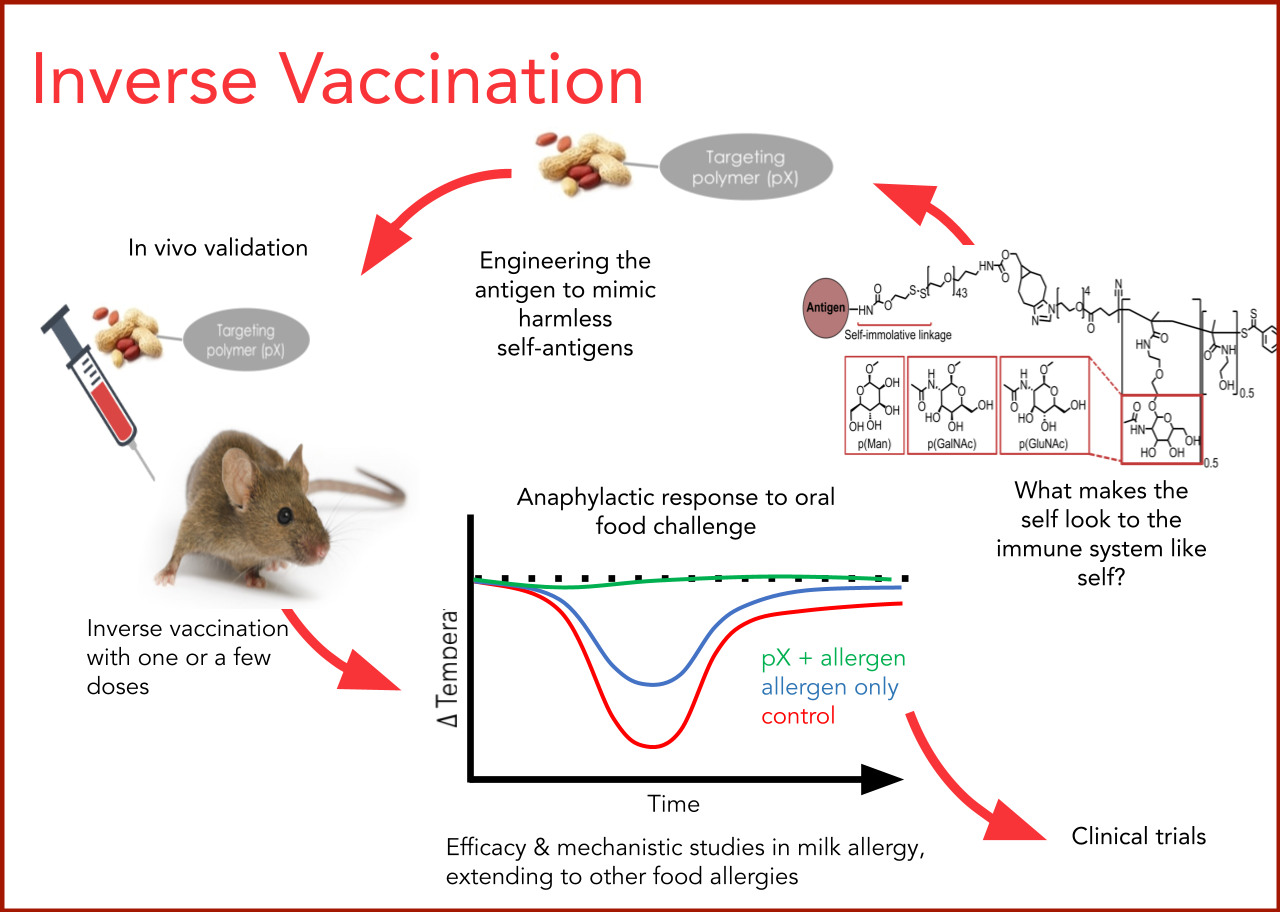
Inverse Food Allergy Vaccines Through Targeted Antigen Delivery
Dr. Jeffrey A. Hubbell, Pritzker School of Molecular Engineering, University of Chicago and Dr. Cathryn R. Nagler, Biological Sciences Division, Pritzker School of Molecular Engineering, University of Chicago, as Co-Principal Investigators
Funded by the Food Allergy Fund
The next phase of this Inverse Vaccine study is to create recombinantly produced immunogenic peanut proteins, Ara h 1, 2 and 6, and to conjugate each of them to the established glycopolymers. The team will investigate mechanisms of protection by analyzing the tolerogenic T and B cell responses (including antibody production) in milk and peanut food allergy models, as well as by measuring changes in the microbial phenotypes. Finally, sublingual administration for inducing sustained non-responsiveness to food allergens will be studied comparatively.
Scroll Down to Click Through Slides
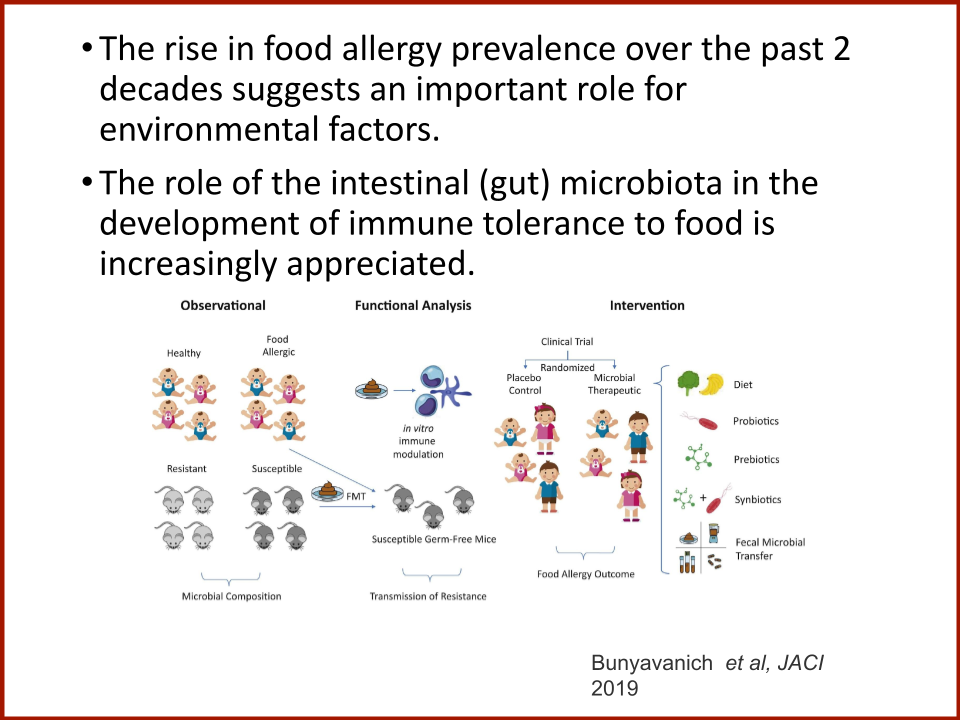
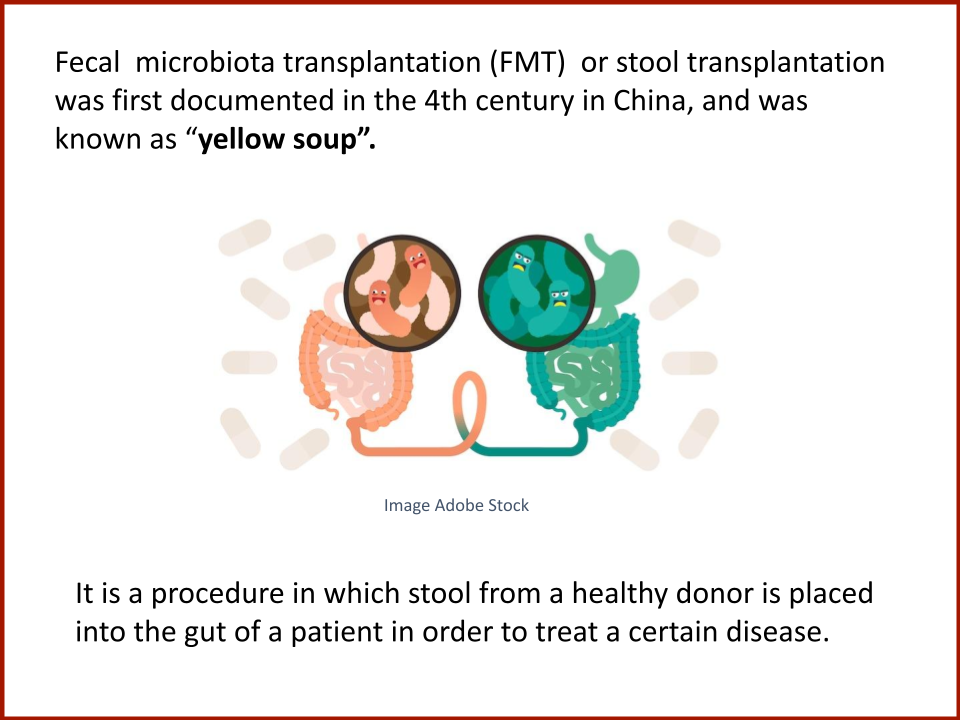
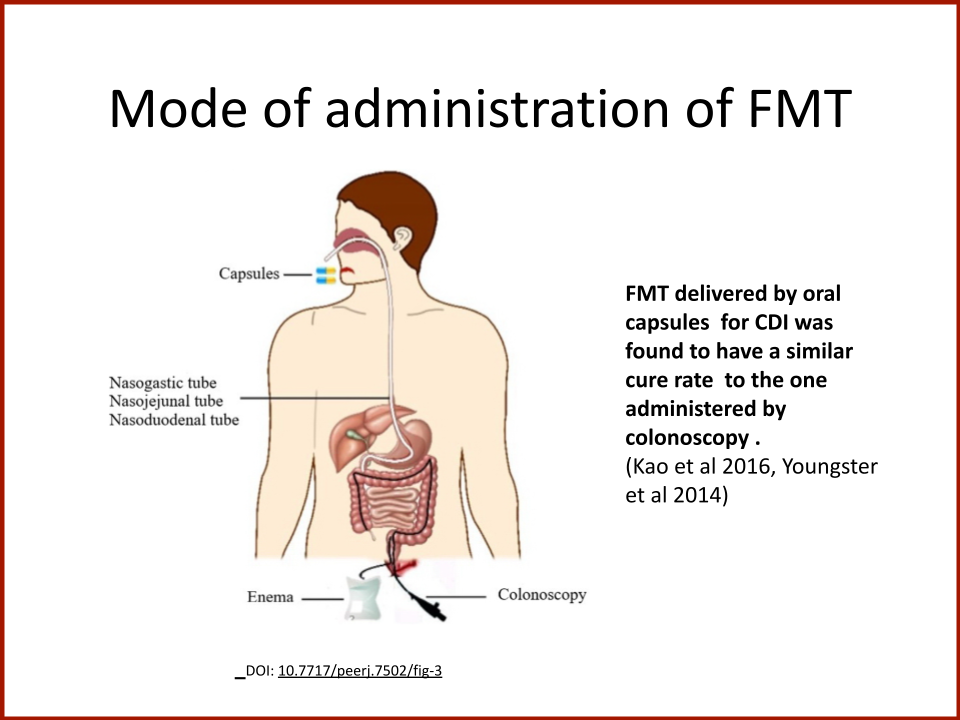
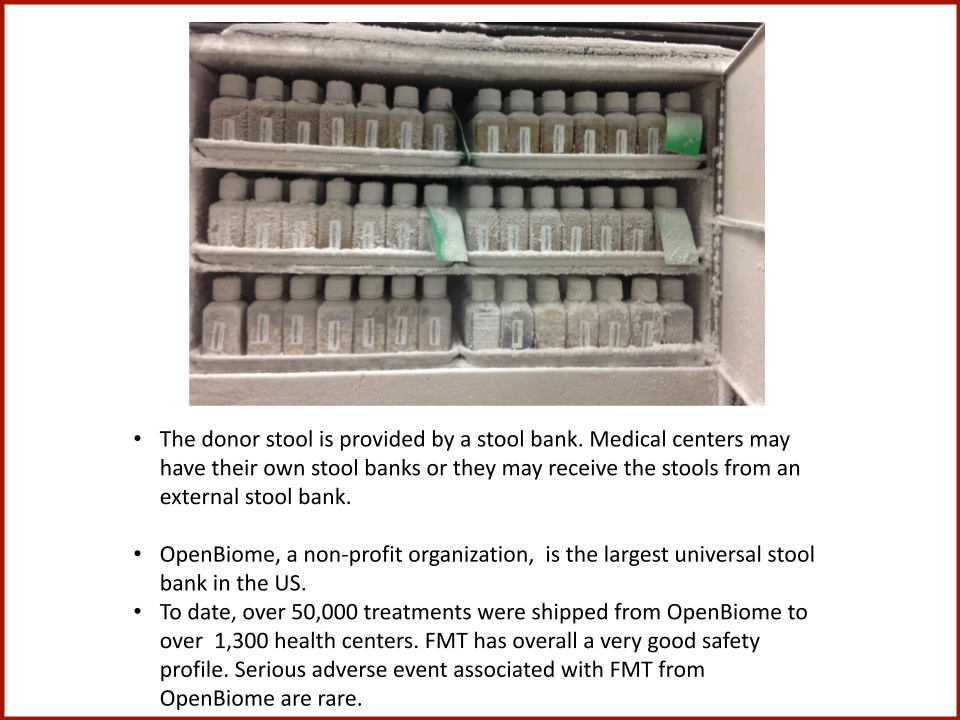
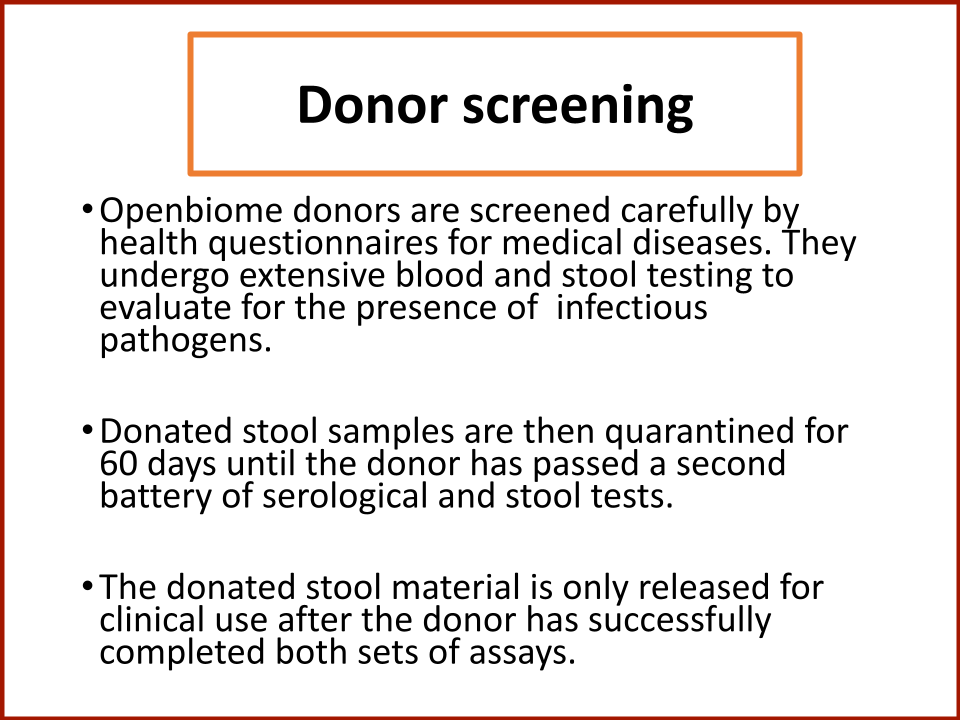
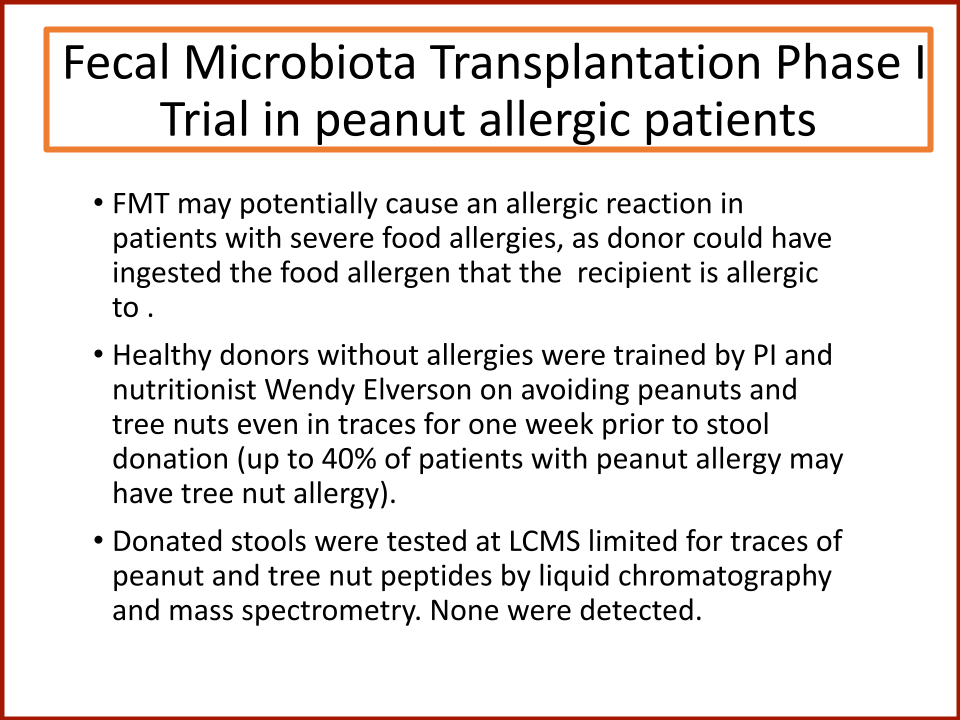
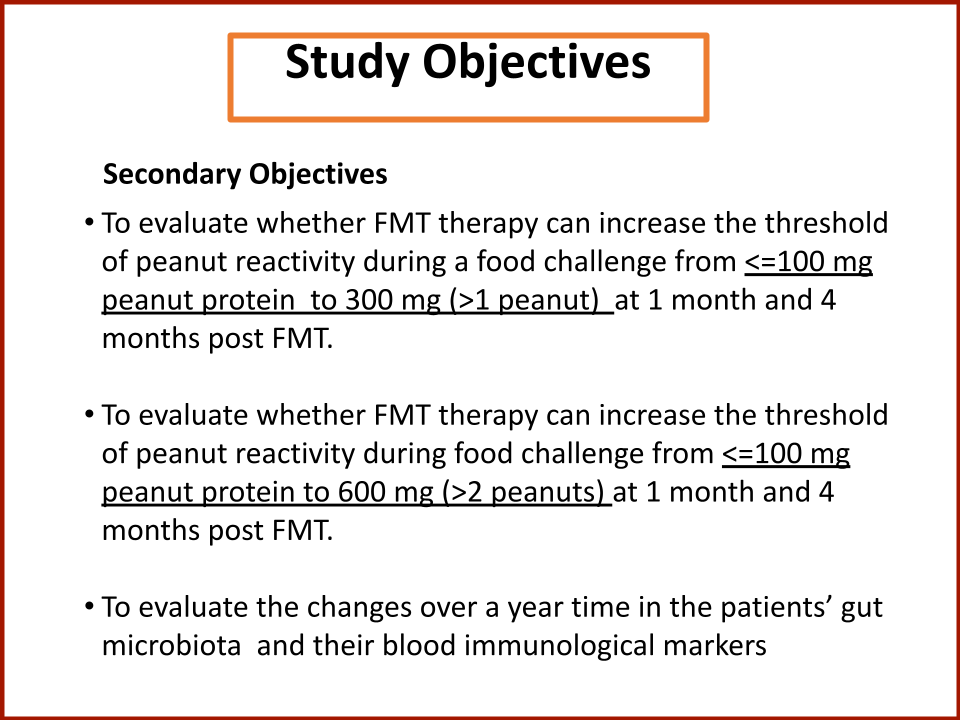
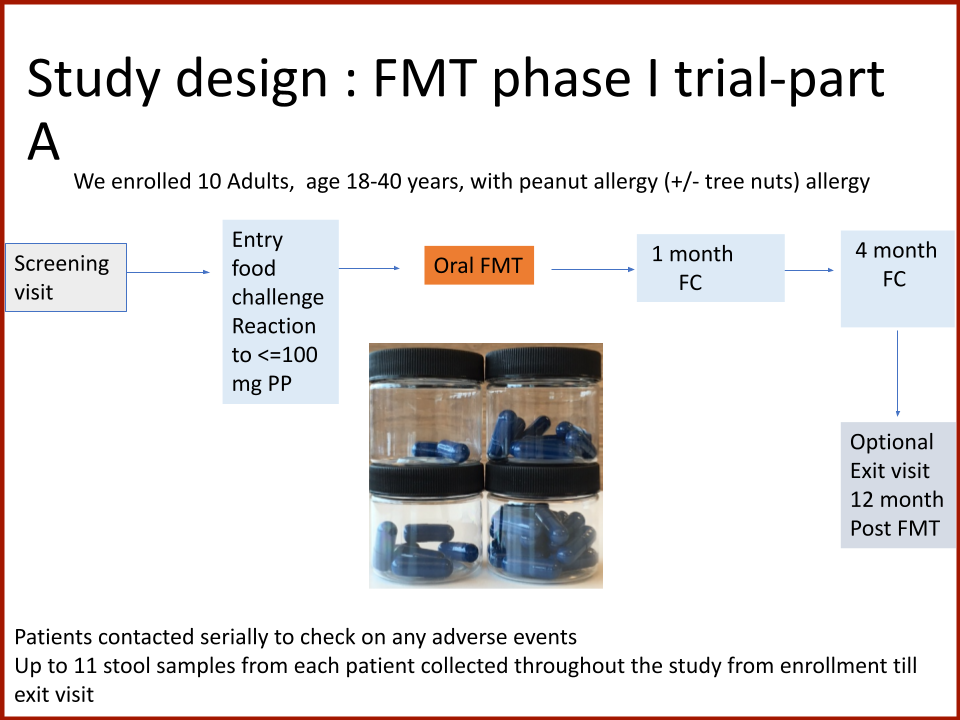
Evaluating the Safety and Efficacy of Oral Encapsulated Fecal Microbiota Transplant in Food Allergic Patients
The first fecal transplant trial ever for food allergies showed enough correlation between non-allergic donor microba and increased food tolerance that researchers wanted to increase their odds for successful food tolerance. Altering the microbiome of food allergic individuals with an antibiotic before receiving “normal'' donor microba might speed up and increase food tolerance even further.
Funded by the Food Allergy Fund
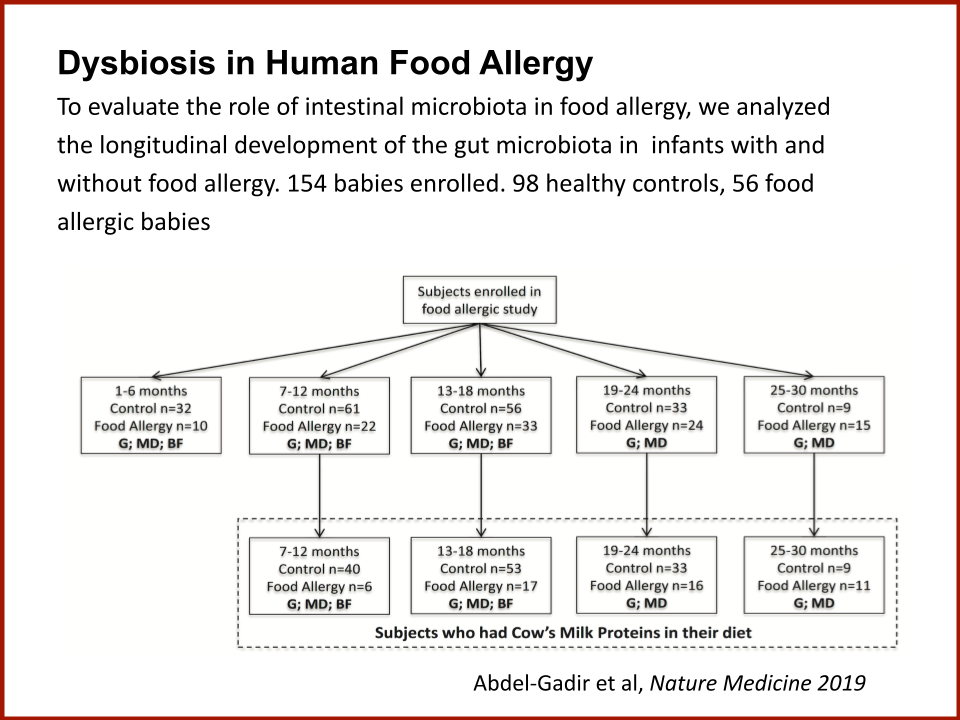
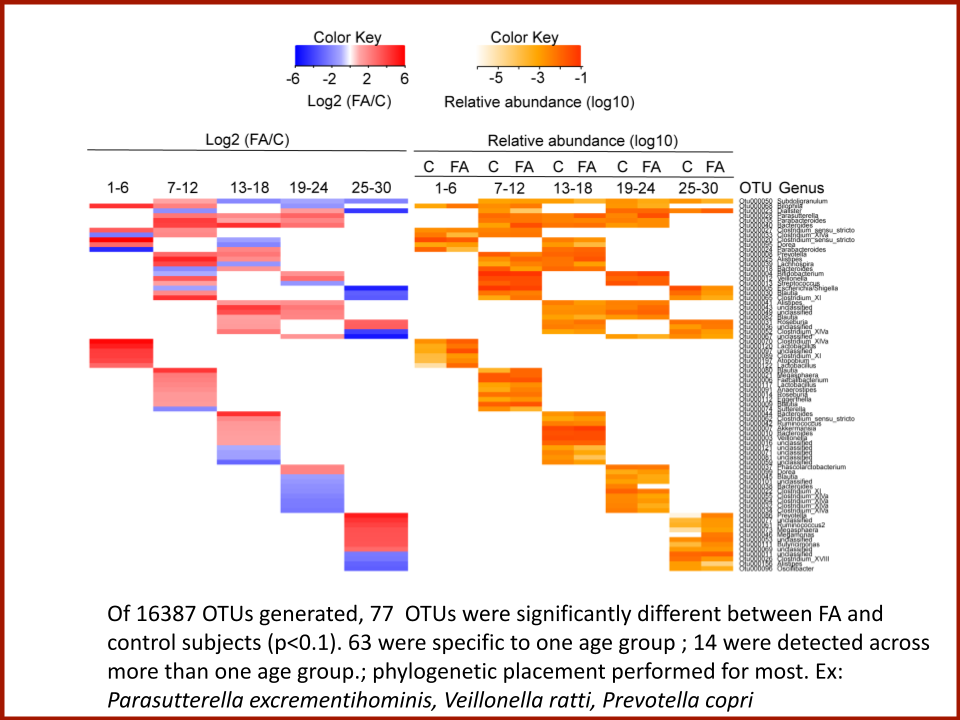
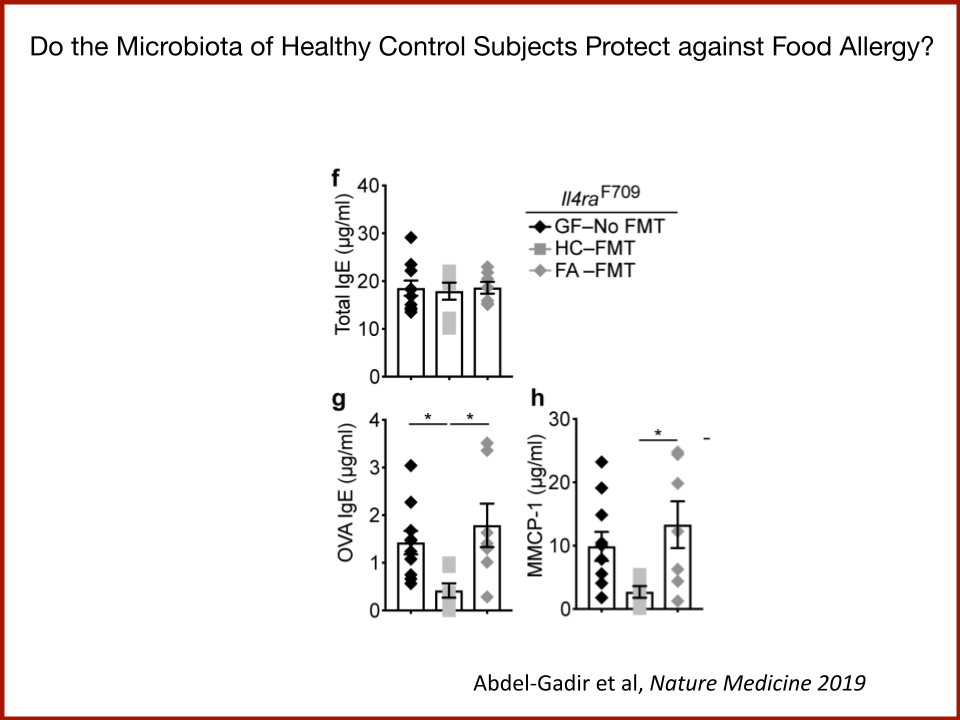
In 2019, the Food Allergy Fund provided crucial support to an additional clinical phase of an investigative clinical study at Boston Children’s Hospital focused on the relationship between bacteria and immune function. Dr. Rachid and Dr. Chatila published groundbreaking discoveries in the journal Nature Medicine on the [link between the microbiome and food allergy][1]. The research team found that transplanting fecal bacteria from healthy infants into allergy-prone mice protected the mice from anaphylaxis. Furthermore, they have identified the immunological pathway by which “good” bacteria signal the body to produce the right kind of immune cells and protect against food allergy.
Based on these cutting edge findings, Dr. Rachid is now leading the first trial in the world to evaluate the safety and efficacy of Fecal Microbiota Transplantation (FMT) in adults with peanut allergy. The preliminary results were promising as the dose at which patients reacted increased significantly in some patients and, the peanut IgE and/or skin test results decreased significantly over time.
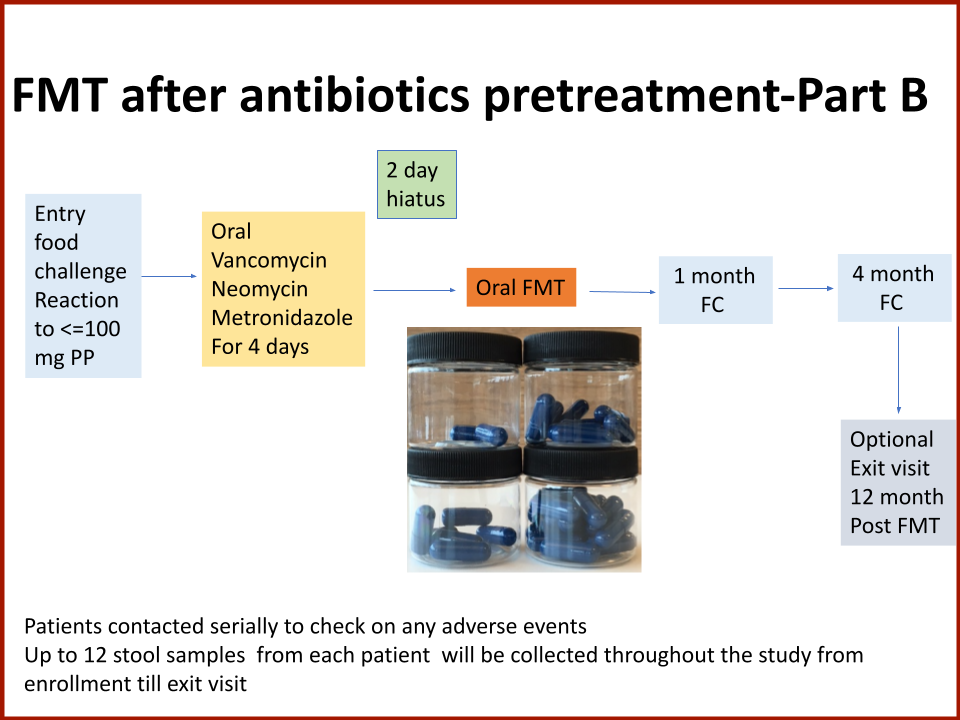
The Food Allergy Fund provided additional support for Dr. Rachid to expand the Phase 1 trial, recruiting additional study participants who received antibiotics for four days prior to their FMT. The goal is to understand if antibiotic treatment before FMT creates a niche for good bacteria, making microbial transplantation possibly more effective.